
They participate in van der Waals interactions, which are essential for the stabilization of protein structures. These residues are normally located inside the protein core, isolated from solvent.

The hydrophobic amino acids include alanine (Ala, A), valine (Val, V), leucine (Leu, L), isoleucine (Ile, I), proline (Pro, P), phenylalanine (Phe, F) and cysteine (Cys). For discussion of OH−π, and CH−O types of hydrogen bonds see Scheiner et al., 2002. We should note here that the side chains of histidine, tyrosine, phenylalanine and tryptophan are also able to form weak hydrogen bonds of the types OH−π and CH−O, using electron clouds within their ring structures. These residues can be found close to the interface between a protein and solvent. The aromatic amino acids tryptophan (Trp), and the earlier mentioned Tyr, as well as the non-aromatic methionine (Met) are sometimes called amphipathic due to their ability to have both polar and nonpolar character. This ability makes histidine useful in enzyme active sites when proton extraction is required by the chemical reaction. The pKa may be modulated by the protein environment in a way that the side chain may give away a proton and become neutral, or accept a proton, becoming charged. When both groups are protonated, the side chain has a charge of +1. It has two –NH group with a pKa value of around 6. Histidine (His), on the other hand, may be both polar and charged, depending on the environment and pH.
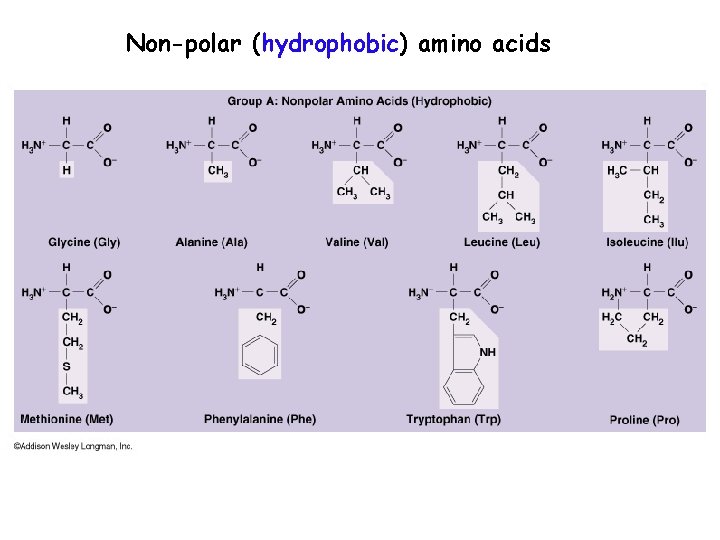
Asparagine (Asn) and glutamine (Gln) are also polar, they carry a polar amide group. This polar group can participate in hydrogen bond formation with another polar group by donating or accepting a proton. For example, serine (Ser), threonine (Thr) and tyrosine (Tyr) are clearly polar since they carry a hydroxyl (-OH) group. When considering polarity, some of the amino acids are straightforward to assign, while in other cases we may encounter disagreements. I hope that at some moment in the future I will complement this compendium by a chapter on metal binding in proteins. Metalloproteins and metal binding is a fascinating area of structural biology. Binding of metal ions in proteins is another function of the negatively charged carboxylic groups of Asp and Glu. For example, charged amino acids in proteins from thermophilic organisms (organisms that live at elevated temperatures, up to 80-90 C, or even higher) often form an extensive network of salt bridges on the surface of these proteins, contributing to their thermostability and preventing denaturation at high temperatures. The so-called salt bridges, which are formed by the interaction between positively and negatively charged amino acid side chains, have been found to be important for the stabilization of protein three-dimensional structure. There are four of them, two basic amino acids, lysine (Lys) and arginine (Arg) with a positive charge at neutral pH, and two acidic, aspartate (Asp) and glutamate (Glu) carrying a negative charge at neutral pH. It is easy to see which amino acids are charged simply because at neutral pH (around 7) they contain a single charge. For example, based on the propensity of the side chain to be in contact with water, amino acids can be classified as hydrophobic (low propensity to be in contact with water), polar and charged (energetically favorable contacts with water). Side chains such as Lys and Arg can thus interact favorably with both polar and nonpolar residues.Each of the 20 most common amino acids has its specific chemical characteristics and its unique role in protein structure and function.


The interactions are hydrophobic with contacts between Val or Ile and the alkyl groups in Arg or Lys. All three stabilize the helix with DeltaG between -0.14 and -0.32 kcal x mol(-1).
#Are hydrophobic amino acids polar or nonpolar free
Experimental circular dichroism results were analyzed with helix-coil theory to calculate the free energy for the interactions. Controls with i, i + 5 spacing have the residues on opposite faces of the helix and are less helical than the test peptides with the i, i + 4 interactions. Partially helical peptides containing pairs of nonpolar/polar residues were synthesized. We observe that the nonpolar/polar pairs Ile-Lys, Ile-Arg, and Val-Lys occur in protein helices more often than expected when spaced i, i + 4. Residues spaced i, i + 4 in alpha-helices are on the same face of the helix, with potential to favorably interact and stabilize the structure. Here we show that nonpolar/polar interactions, namely Val or Ile bonding to Lys or Arg in alpha-helices, can in fact be stabilizing. A simplistic, yet often used, view of protein stability is that amino acids attract other amino acids with similar polarity, whereas nonpolar and polar side chains repel.


 0 kommentar(er)
0 kommentar(er)
